Isotopes - two or more atoms of the same element having different number of neutrons. For what you asking i'm not 100% sure, but i do know that the first energy level can hold 2 electrons, and the second level can have 8. Hope it helps:). Isotopes are atoms that contain the same number of protons, but they have different number of neutrons. This also could be understood as isotopes are those atoms which have the same proton number (atomic number) but a different nucleon (mass) number. Note: all atoms that are of the same element contain the same number of Protons.
- ITs Z Type And Trust Me It Is!! I Put A And I Got It Wrong!!!
- Isotopes Of The Same Element Have The Same Number Of Neutrons
- Isotopes Of The Same Element Have The Same Number Of What
How do different isotopes of the same element differ?
2 Answers
Explanation:
Two or more than two kinds of atom which have same proton number but different neutron number so that the mass number changes e.g

Isotopes differ in the number of neutrons in the nucleus
Explanation:

all isotopes have the same number of protons and the same number of electrons. Because the electron structure is the same isotopes have the same chemical properties.
What is different is the number of neutrons, The different number of neutrons all cause a difference in the atomic weight or mass of the atoms. The difference in the ratio between the number of protons (the number of protons stay the same) and the number neutrons creates a difference in the nuclear reactions of the isotopes.
What is different about isotopes is the number of neutrons. The difference in the number of neutrons also causes a difference in the mass and nuclear reactions of the isotopes.
Related questions
Isotopes are variants of a specific chemical element. For example, uranium-238, uranium-235 and uranium-234 are three isotopes of the element uranium. The listed numbers are the mass number and each isotope has a different mass number. Download kindle book as pdf mac. This number is calculated by adding the amount of protons and neutrons that the isotope contains in the nucleus and it is one of the differences between isotopes from the same element. Let’s find out some of the most common similarities and differences between these isotopes.
Bitcoin mining software mac download. Similarities between isotopes of the same element
Each isotope of the same element is identical in most ways including having the same number of protons and electrons.
Cisco anyconnect not able to establish a connection without. Note: Make sure that port 443 is not blocked so the AnyConnect client can connect to the ASA. When a user cannot connect the AnyConnect VPN Client to the ASA, the issue might be caused by an incompatibility between the AnyConnect client version and the ASA software image version.
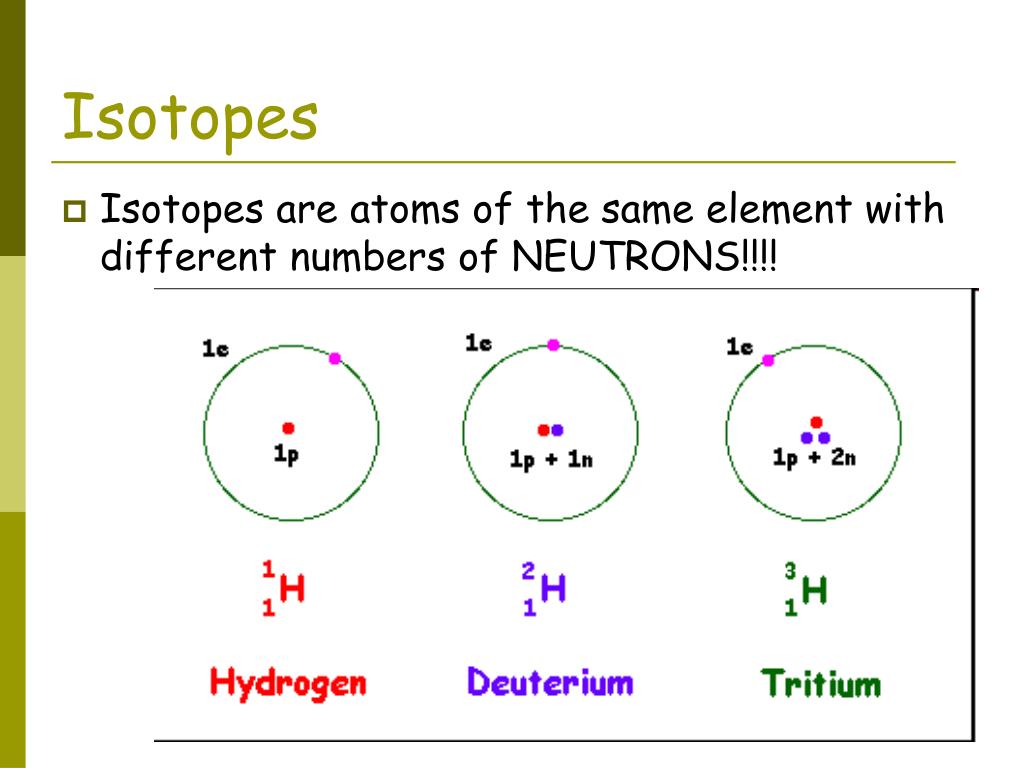
Differences between isotopes of the same element
Each isotope of the same element contains a different number of neutrons and this is the main difference between isotopes of the same element. The isotopes will also have a slightly different atomic mass because of the different number of neutrons. Radioactive (unstable) isotopes will also have different half lives (rate of decay). They may also a different type of decay and daughter isotope (daughter product), which is the product left over after radioactive decay.
ITs Z Type And Trust Me It Is!! I Put A And I Got It Wrong!!!
Did you know?
Many of the elements have at least one stable isotope and a number of unstable (radioactive) isotopes. However, certain elements have no stable isotopes at all. This includes the elements technetium, promethium and every element after lead.
Many radioactive isotopes have very important uses in fields such as geology (radiometric dating), medicine (nuclear medicine), astronomy (radiometric dating), fire prevention (smoke detectors), food preservation (irradiation) and pest control (irradiation).
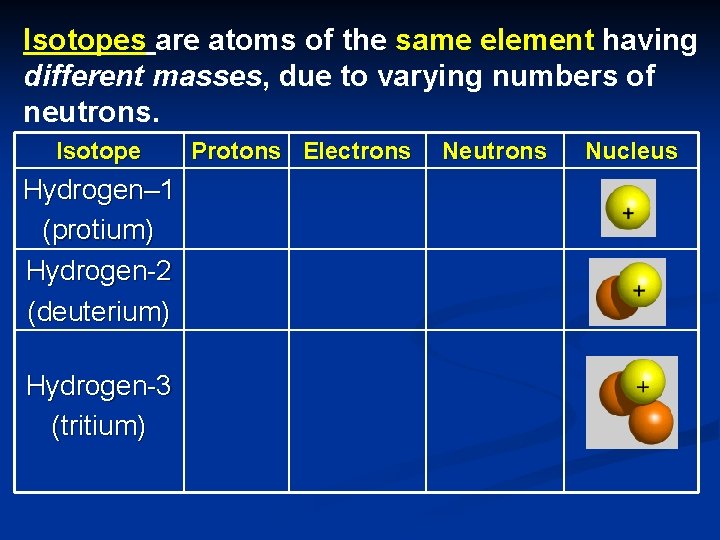
Isotopes Of The Same Element Have The Same Number Of Neutrons

Isotopes Of The Same Element Have The Same Number Of What
Related Articles
